Draw the shape of the #ClF_2^+# ion, including any lone pairs, and name the shape made by the atoms? Predict the bond angle in the ion?
1 Answer
Dec 12, 2017
The molecular shape is "bent", with a theoretical bond angle of 109.5°.
Explanation:
Step 1. Draw the Lewis structure
We put the least electronegative atom in the centre.
We have
This gives us
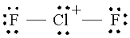
(Adapted from Quora)
Step 2. Determine the shape of the ion
The central atom has two lone pairs and two bonding pairs (four electron domains).
According to VSEPR theory, this predicts a tetrahedral electron geometry.

The molecular geometry includes only the bonding pairs, so the molecular shape is bent, with a theoretical bond angle of 109.5°.
(The actual

