What is the Lewis structure for resonance form of #ClO_2^-#?
1 Answer
Dec 16, 2017
Well we has got................
Explanation:
And so....we gots
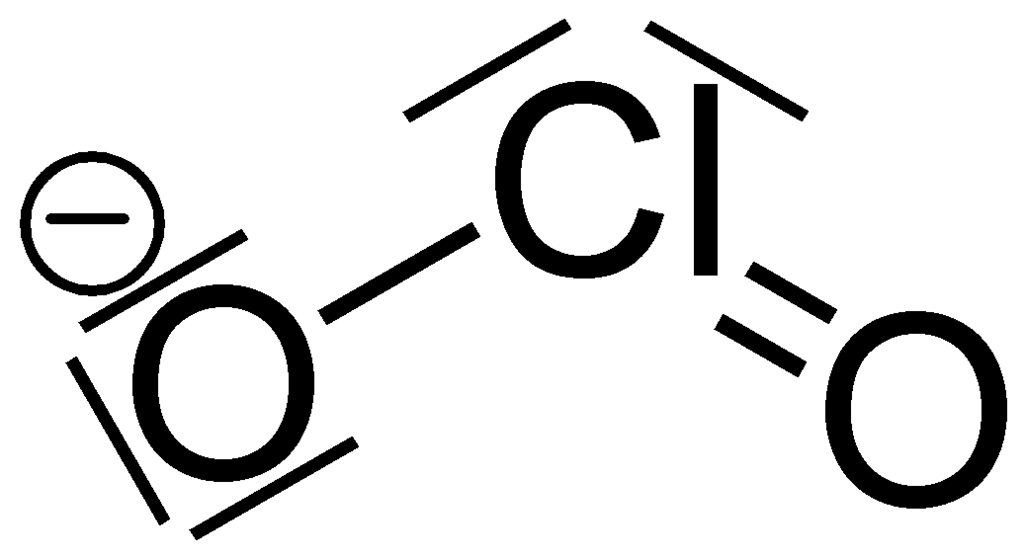
The electronic geometry around chlorine is tetrahedral. The molecular geometry is bent with

