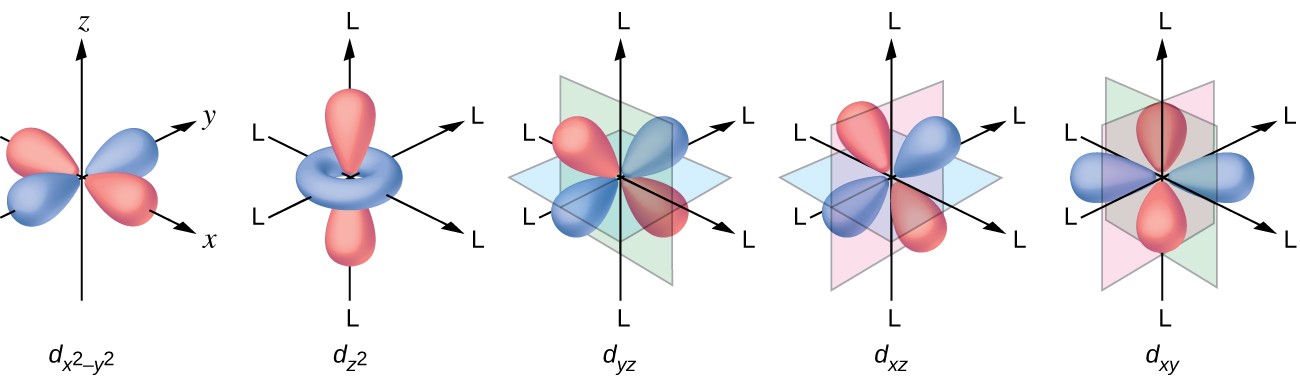
How many orbitals are found in a d subshell?
1 Answer
Dec 29, 2017
Explanation:
The number of orbitals present in a given subshell is given by the number of values that the magnetic quantum number,
In turn, the possible values that the magnetic quantum number can take depend on the identity of the angular momentum quantum number,
#m_l = { -l, -(l-1), ..., - 1, 0, +1, ..., +(l-1), +l}#
Now, the
#l = 2#
This means that for a
#l = 2 implies m_l = {-2, -1, 0, +1, +2}#
You can thus say that the