What kind of chemical bond is formed when an equal exchange of electrons occurs?
1 Answer
Jan 8, 2018
A covalent bond is when two atoms share valence electrons to obtain a full valence shell, which translates into more stability.
For instance, consider
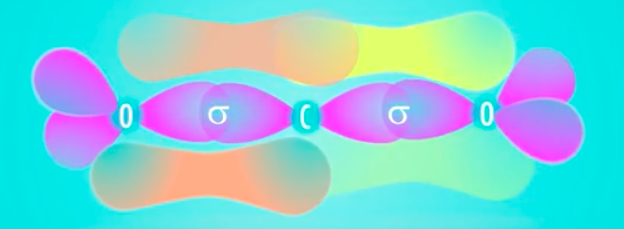
or more simply,

In this configuration, each atom has an octet (since the oxygen atoms don't have formal charges, it's conventional to assume they each have two lone pairs in addition to the double bonds).
In the orbital diagram, the carbon atom has two

