Question #11a21
1 Answer
Jan 12, 2018
see below
Explanation:
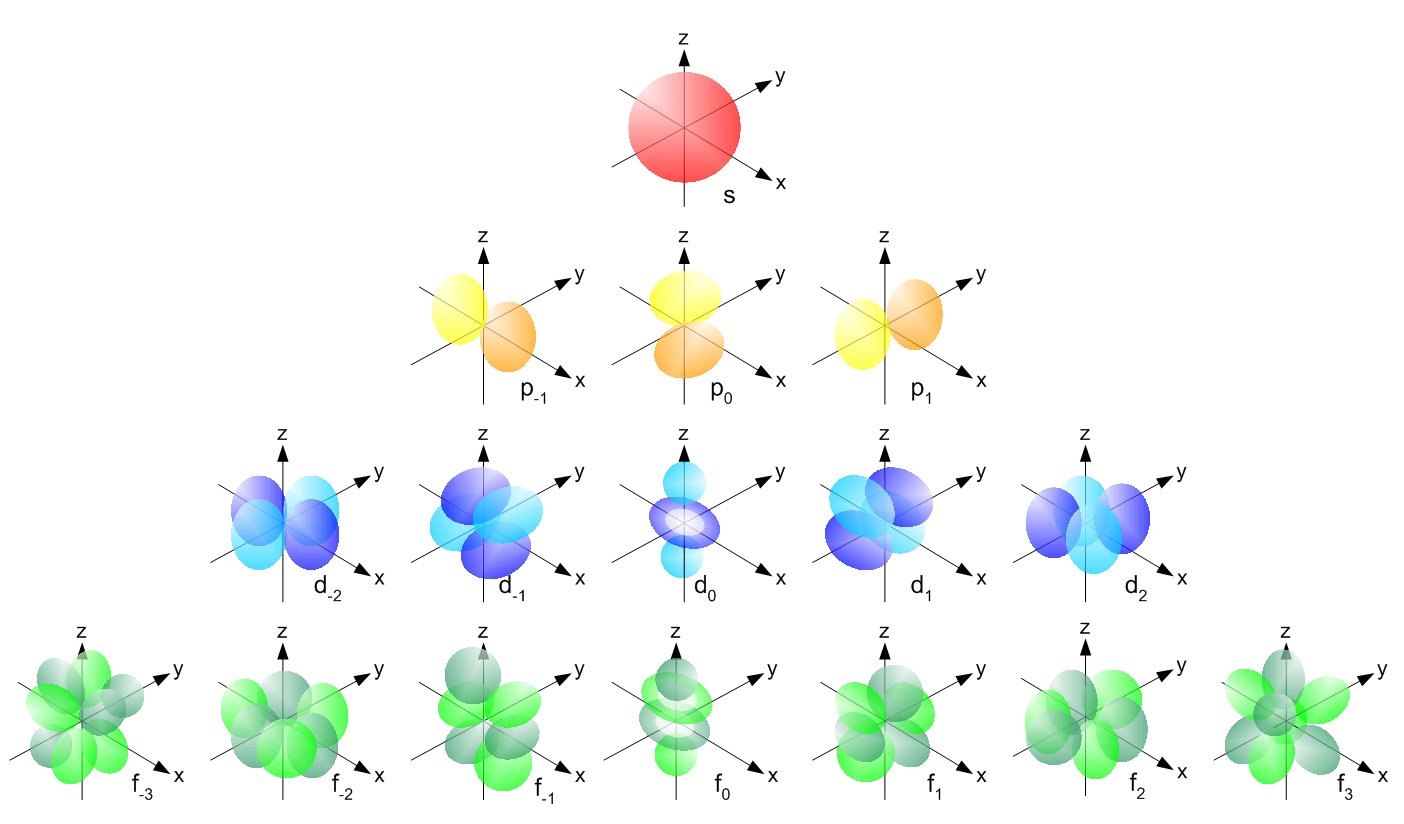
electrons in atom does not follow a circular path (Bohr) neither an elliptical path (Sommerfield) but an irregular motion (according to quantistic mechanical) in which the sum of potential and kinetic energy is constant. Their positions during their movements occupy some particular spazial zones called orbitals and caracterized by particular forms that you ca easily find on internet. According with Haisemberg you can't know at the same time their positions and their speeds