What happens to the reaction rate as a reactant gets used up?
1 Answer
Mar 29, 2018
It gets slower...
Explanation:
We know that the more concentrated the product is, the faster the reaction rate happens. This is because more of the product's molecules are able to collide with the other molecules to form a reactant.
So, when a reactant gets used up, its concentration decreases, and so the total reaction rate decreases.
Have a look at this diagram:
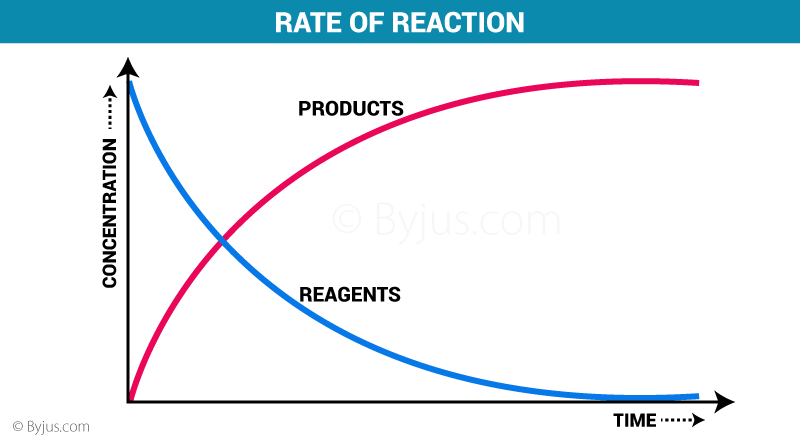
As you can see here, more products equal less reagents (reactants), and so the reaction rate decreases.

