How does the 3s orbital differ from the 2s orbital?
1 Answer
Simply put - it's larger.
Explanation:
Consider the difference between a 1s and 2s orbital...
1S ORBITAL:
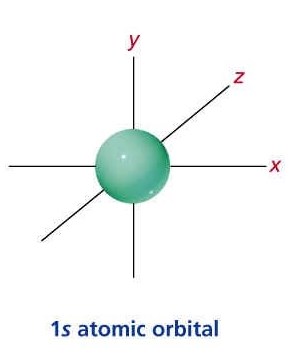 http://personal.monm.edu/gebauer_peter/chem_230/Lect_pages/Bruice_jpgs&pgs/s_orbitals.htm
http://personal.monm.edu/gebauer_peter/chem_230/Lect_pages/Bruice_jpgs&pgs/s_orbitals.htm
The "1" shows the orbital is within the energy level closest to the nucleus while the "s" describes the shape of the orbital (spherical for S).
2S ORBITAL:
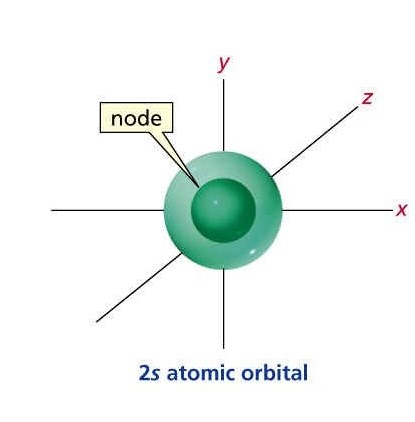 http://personal.monm.edu/gebauer_peter/chem_230/Lect_pages/Bruice_jpgs&pgs/s_orbitals.htm
http://personal.monm.edu/gebauer_peter/chem_230/Lect_pages/Bruice_jpgs&pgs/s_orbitals.htm
The whole idea/design doesn't change really, only the possible areas at which the Electron might be in is greater.
Same goes for the 3s orbital, 4s, etc...
Just larger orbitals with less and less possibility of finding the electron in a more "simple and confined" space
There obviously much more gory detail to this, but this is the basic rundown.

