What can decrease the activation energy needed to start a reaction?
1 Answer
May 16, 2018
An alternative activation pathway....
Explanation:
...and that means a CATALYST, which (somehow) combines with the substrate and mediates its reaction... Catalysts lower (or raise) activation energies. They are not magic and CANNOT affect the thermodynamics of a reaction....
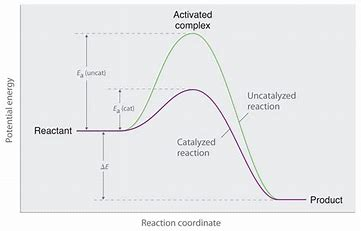
Is it clear from the diagram how the activation energy is reduced....and yet

