What is an achiral carbon?
1 Answer
This is a carbon centre that is not of the form
Explanation:
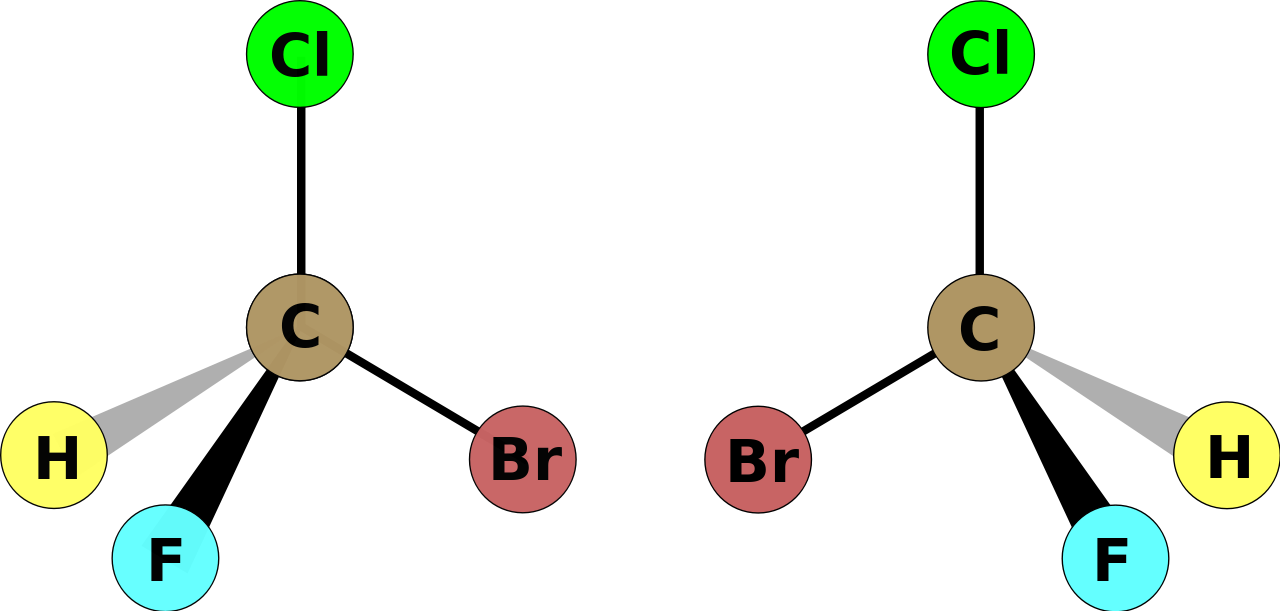
The so-called stereoisomer on the LEFT (left-handed with respect to carbon) is geometrically different to the stereoisomer on the RIGHT. The two isomers are optical isomers that CANNOT be superimposed. With a model (and you should be using a model!) the interchange of any two groups results in the enantiomer, interchange again and you get the enantiomer of an enantiomer, i.e. the original isomer.
See here and links for more explanation and examples....
For a more practical example, consider the pair of shoes that you put on for work. Can you put your left shoe on your right hoof? Will it be comfortable? But how is your left foot different from your right hoof. They are (usually) identical structurally...

