Does the atomic volume rise with the increasing atomic mass?
1 Answer
Interesting proposition....
Explanation:
And the answer is not necessarily. We specify ATOMIC volume, and reasonably, such volume is the volume swept out by the valence electrons in a given ATOM.
Now it is well known that atomic size, i.e. atomic radius, INCREASES down a Group, a column of the Periodic Table. Why so? Because the valence electrons build on an inner core of electronic the which already possess a more or less fixed electronic structure.
On the other hand, ACROSS the Period, across a row of the Periodic Table, from LEFT to RIGHT, AS WE FACE the Table, atomic size, i.e. radius, DECREASES, given that as the nuclear charge,
And so let us look at some data with respect to atomic size...
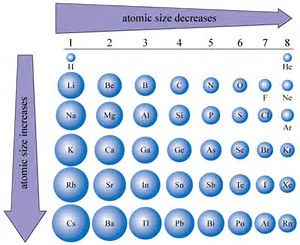
Do these support the given argument?
Sorry for all the SHOUTING....but I wanted to emphasize certain properties.

