How do you use electronegativity values and the chemical formula of a substance to tell if the substance is nonpolar covalent, polar covalent, coordinate covalent, or ionic.?
1 Answer
You use these differences VERY selectively, and sometimes arbitrarily...
Explanation:
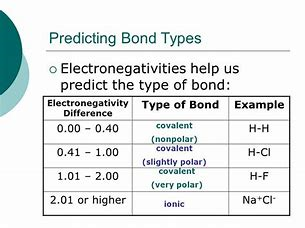
I myself find these tables a bit mickey mouse, inasmuch FIRST you have to have Tables of electronegativity in order to make the assessment of
An equally important distinction to make is whether the substance is molecular or NON-MOLECULAR. Non-molecular materials, e.g. carbon, salts, silica, have INTRINSICALLY HIGH melting and boiling points in comparison to molecular materials.

