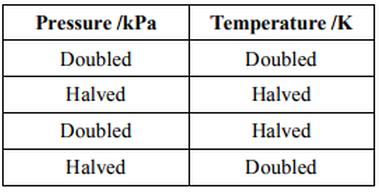
Which change in conditions would increase the volume of a fixed mass of gas?
1 Answer
Jun 8, 2018
Increase temperature
Explanation:
We can use the Ideal Gas Equation to solve this question:
Hre
#P# is pressure in#"Pa"# #V# is volume in#"m"^3# #n# is number of moles of gas#R# is the universal gas constant,#"8.31 J/K mol"# #T# is temperature in Kelvin
In your scenario, when mass is fixed, the number of moles will be fixed, too. So we can combine both constant terms
where
Now, since we want to work out how volume changes, let's put
So from the equation, we can deduce that in order to increase volume, we can either increase temperature
Hope this helps!


