What would cause an atom to have a low electronegativity value?
2 Answers
A reduced nuclear charge....
Explanation:
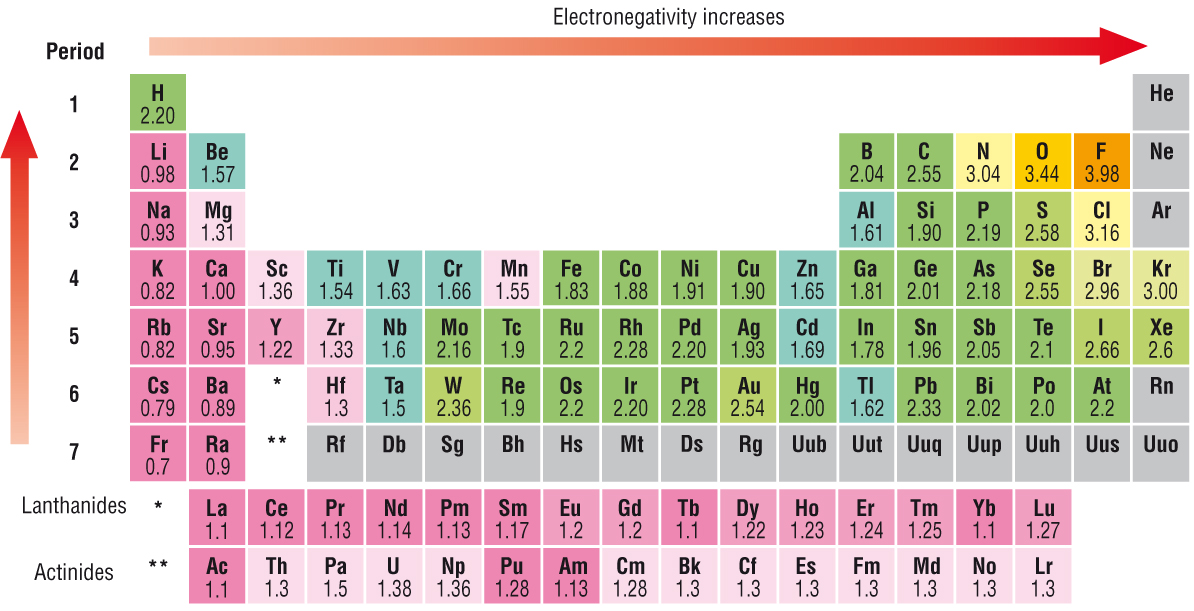
Electronegativity INCREASES ACROSS the Period from left to right as we face the Table, but DECREASES DOWN the Group. Why so? As always, it is a competition between (i) nuclear charge, i.e. the
And elements to the LEFT of the Periodic Table, the alkali metals, and the alkaline earths, should have intrinsically low electronegativities, given that the nuclear charge is well shielded by the intervening electronic shells...
Does this scale of electronegativity support or detract from the given argument...?
A lack of positive charge compared to the distance of the electrons from the nucleus.
Explanation:
Sodium has a lower electronegativity than Chlorine in the same period. The average distance of the electron density of the electron orbitals is the same for Sodium and Chlorine. Sodium has only 11 protons pulling on its electron density while Chlorine has 17 protons. This allows Chlorine to have a greater pull or electronegativity that Sodium ( It also has a smaller atomic radii due to the greater pull)
Sodium has a higher electronegativity than Potassium. Both Sodium and Potassium are in the same family. Potassium's valance electrons are in the fourth shell while Sodium's valance electrons are in the third shell and are much closer to the nucleus. Because Potassium's electron density is further away from the positive nucleus the protons have less pull. Thive more pull on the electron density the greater the electronegativity.
The further the electron density is from the positive nucleus the lower the electronegativity, The smaller the positive charge on the nucleus the lower the electronegativity. If an atoms is larger with more electron shells it will have a lower electronegativity, If the nucleus has fewer protons in the same period it will have a lower electronegativity.

