How do London dispersion forces relate to the boiling point?
1 Answer
Jul 10, 2018
Is not the greater the dispersion force, the greater the boiling point?
Explanation:
Dispersion forces results from the transient polarization of electron clouds, and the momentary formation of dipoles. And so let us interrogate the boiling points of the Noble Gases, for which the
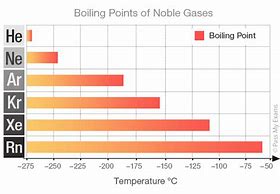
The larger Noble gases have more electrons, and thus more opportunity for dispersion forces. Do not the progressively increasing boiling points illustrate the proposed

