How are alkenes named?
1 Answer
Jul 23, 2018
Well, what is standard operating procedure....
Explanation:
For a given olefin, as normal we identify the LONGEST carbon chain:
...the numbering is unambiguous here, and so we gots
But of course both rings and olefinic bonds, sites of unsaturation, introduce another level of complexity...I could have...
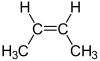
...or....
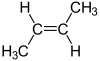
Because the geometry is different while the carbon-carbon connectivity is the same, these are geometric isomers, that have different chemical and physical properties...

