Why does ice have a different density than liquid water?
1 Answer
It has to do with how the
Explanation:
The difference between the densities has to do with how
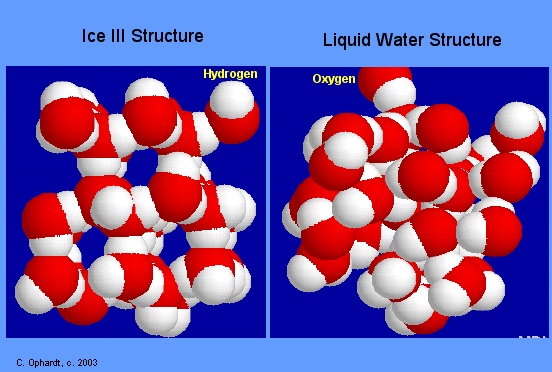
From the image above, notice how the ice molecules (image on left) are connected in a more rigid manner. The liquid water molecules (image on the right) on the other hand are connected closer to each other.
This means, in a given volume, let's just say
So, if we were to look at the formula of density:
Since we are looking at same volume,
That's why, density of water is higher than density of ice.

