1s^1 2s^1 is an electron configuration of an excited atom. What is the identity of the atom and what is its ground state configuration?
1 Answer
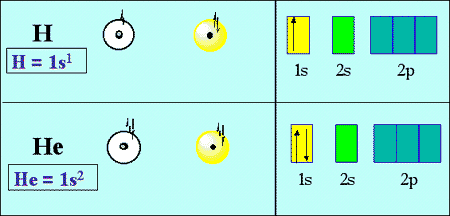
Helium.
Explanation:
For starters, your atom contains
You can say that because a neutral atom has equal numbers of protons inside the nucleus and of electrons surrounding the nucleus, regardless if the atom is in its ground state or not--keep in mind that this is true when dealing with atoms, not with ions.
"2 electrons " -> " 2 protons " -> " atomic number"color(white)(.)(Z) = 2 " "-> " He"
Now, for a given atom, an excited state is characterized by the fact that one or more electrons occupy higher energy levels than they do in the ground state.
You can spot which electrons have been promoted to a higher energy level by looking at the electron configuration of the atom.
In your case, you have
color(blue)(1)s^1 color(red)(2)s^1
Now, you should know that electron configuration of an atom in its ground state is determined by Aufbau's Principle, which states that electrons must occupy orbitals in order of increasing energy.
As you know, the second energy level, denoted by the principal quantum number
Now, notice that your atom has
As you know, the first energy level can hold
This implies that the
Since the second electron was added to the
Consequently, you can say that the ground state configuration of a helium atom should look like this
"He: " color(blue)(1)s^2
 https://socratic.org/questions/what-is-the-electron-configuration-for-helium
https://socratic.org/questions/what-is-the-electron-configuration-for-helium

