Fluorine (#F_2#) is a homonuclear diatomic molecule that has 18 electrons (9 from each #F# atom) - out of which 14 are valence electrons (7 from each #F# atom)..
Molecular Orbital Theory predicts the distribution of electrons in a molecule.
Now, the molecular orbital (MO) diagram for #F_2# is this:
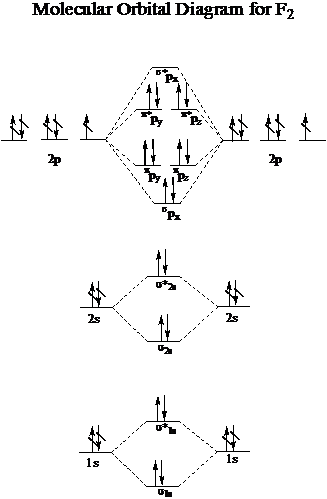
#F_2#'s complete electron configuration with respect to its bonding and antibonding orbitals is:
#F_2#:#sigma_(1s)^2 sigma_(1s)^(**2) sigma_(2s)^2 sigma_(2s)^(**2) sigma_(px)^2 pi_(py)^2 pi_(pz)^2 pi_(py)^(**2) pi_(pz)^(**2)#
Bond order is defined as the difference between the number of bonding electrons divided by 2, and the number of antibonding electrons divided by 2; we can see that #F_2# has 10 electrons in its bonding orbitals ( 2 in #sigma_(1s)#, 2 in #sigma_(2s)#, 2 in #sigma_(px)#, 2 in #pi_(py)#, and 2 in #pi_(pz)#) and 8 electrons in its antibonding orbitals (2 in #sigma_(1s)^(star)#, 2 in #sigma_(2s)^(star)#, 2 in #pi_(py)^(star)#, and 2 in #pi_(pz)^(star)#) so its bond order is
#BO_(F_2) = 1/2 * 10 - 1/2 * 8 = 1#
For #F_2^+#, the number of electrons is #18 - 1 =17#, which will determine its electron configuration to be
#F_2^-#: #sigma_(1s)^2 sigma_(1s)^(**2) sigma_(2s)^2 sigma_(2s)^(**2) sigma_(px)^2 pi_(py)^2 pi_(pz)^2 pi_(py)^(**2) pi_(pz)^(**1)#
One electron is now unpaired in its #pi_(pz)^(star)# antibonding orbital. The #F_2^+# molecule will now have 3 more electrons in its bonding orbitals, which will determine the bond order to be
#BO_(F_2^+) = 1/2 * 10 - 1/2 * 7 = 3/2#
For #F_2^-#, the number of electrons will be #18 +1 = 19#, and its electron configuration will be
#F_2^-#: #sigma_(1s)^2 sigma_(1s)^(**2) sigma_(2s)^2 sigma_(2s)^(**2) sigma_(px)^2 pi_(py)^2 pi_(pz)^2 pi_(py)^(**2) pi_(pz)^(**2) sigma_(px)^(**1)#
One electron is now unpaired in the previously-unoccupied #sigma_(px)^(star)# - there will now be 10 electrons in its bonding orbitals and 9 electrons in its antibonding orbitals
#BO_(F_2^-) = 1/2 * 10 - 1/2 * 9 = 1/2#
Since #F_2^+# has the largest BO, it will required more energy to dissociate than #F_2# (BO = 1) and #F_2^-# (BO = 0.5), therefore it will have the strongest bond.


