Question #898c4
1 Answer
The molarity of your diluted solution will be
Basically, you know that you must take a sample of a stock solution and dilute it to a new volume. You'll take 65.0 mL of this 0.925 M-stock solution and dilute it to 185.0 mL.
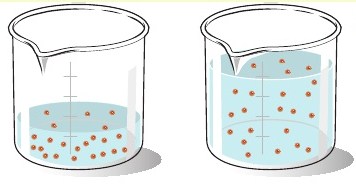
Even before doing any calculations, try and predict what's going to happen to the concentration. As you know, concetration is defined as moles of solute, in your case sodium sulfate, per liter of solution.
The key to the concept of dilutions is to always keep in mind that the number of moles remains constant, i.e you'll have the same number of moles of sodium sulfate in the 185.0-mL solution, as you had in the 65.0-mL solution.
But since the volume increased, the result will be a decreased concentration
Mathematically, this is written as
Plug your values into this equation and you'll get
The difference in volume between your initial solution and your diluted one will be pure water, which means that the added volume of water will be

