Question #82b49
1 Answer
You need
So, what you essentially have to do is work backwards from a diluted solution of sodium hydroxide to determine the volume of the stock solution used to make this solution.
The key concept when dealing with dilution calculations is that the number of moles of solute, in your case sodium hydroxide, remains unchanged.
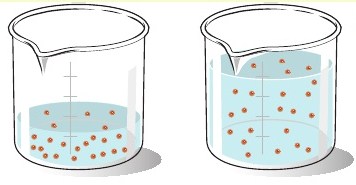
The diluted solution will have the same number of moles of sodium hydroxide as the stock solution. Mathematically, this is written as
Plug your values into the above equation and solve for
So, if you take 65.5 mL of a 5.5-M sodium hydroxide solution and add enough water to make the volume equal to 300 mL, the concentration of the resulting solution will be 1.2 M.
The answer should indeed be rounded to one sig fig because of the number of sig figs in 300 mL; as a result, the volume will be

