Question #a6f93
1 Answer
Aug 28, 2015
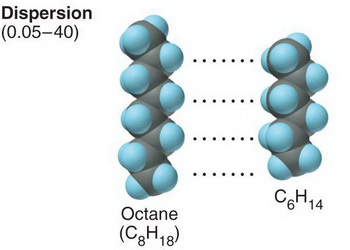
Dispersion forces increase with contact area because there are more fluctuations in electron density around the surface.
Explanation:
You have learned that London dispersion forces are caused by a temporary fluctuation of electron density in one molecule that induces a temporary dipole in a nearby molecule.

Large molecules have big surface areas and more electrons.
There are more fluctuations in electron density in different parts of the molecule.
 Surface area
Surface area
(from www.studyblue.com)
The small dispersion forces around the molecule add up to cause stronger attractive forces with its neighbours.

