Question #08502
1 Answer
Here's what I get.
Explanation:
Question 1
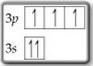
Both electrons in the
This violates the Pauli Exclusion Principle: No two electrons in the same orbital can have the same spin.
Question 2
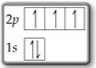
You have no electrons in the
This violates the Aufbau Principle: When adding electrons to an atom, you put them in the lowest-energy orbitals available.
Question 3
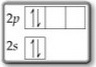
You have two electrons in one
This violates Hund's Rule: There must be one electron with the same spin in each orbital of the same energy before you can put two in the same orbital.
Question 4

The electrons in the half-filled
This violates Hund's Rule: There must be one electron with the same spin in each orbital of the same energy before you can put two in the same orbital.
Question 5
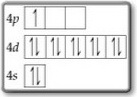
You filled the
This violates the Aufbau Principle.
 www.ck12.org
www.ck12.org


