Question #18bdd
2 Answers
That depends on the substance you're working with. The relationship between mass and volume goes through density, which is used express the mass of a substance per unit of volume.
Take, for example, water. Water has a density of approximately
Here's how other substances compare with water (note that
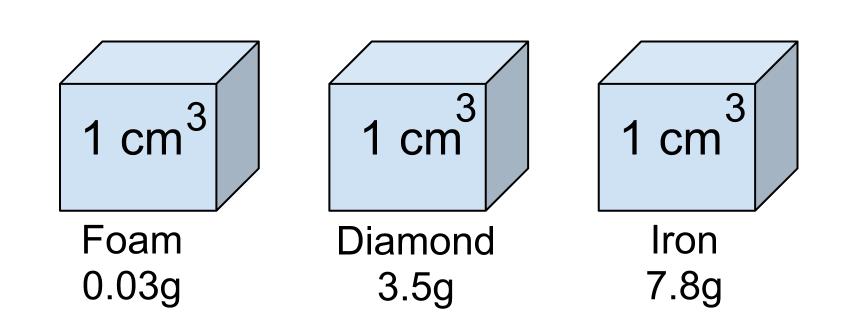
Equal volumes of foam, diamond, and iron will have different weights because these substances have different densities. So, whenever you have to determine the mass of 1 mL of a substance, use its density.
its depends on density
density of any fluid is mass per unit volume
for example- density of water is 1g= 1ml.

