What type of hybridization does the central atom in #"SF"_4# have?
1 Answer
In sulfur tetrafluoride, the central atom is
Explanation:
To determine the orbital hybridization of the sulfur atom, which is the central atom in sulfur tetrafluoride, start by darawing the molecule's Lewis structure.
The molecule will have a total of 34 valence electrons, 6 from sulfur, and 7 from each of the four fluorine atoms.
The sulfur atom will form single bonds with each of fluorine atoms. These bonds will account for 8 of the 34 valence electrons. Each fluorine atom will have 3 lone pairs of electrons in order to have a complete octet.
These lone pairs will use up 24 valence electrons, bringing the total of used valence electrons to 32. The remaining two electrons will be placed on the sulfur atom as a lone pair.
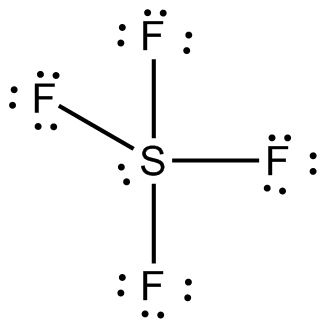
Now focus on the sulfur atom. Notice that it gets a total of 10 electrons, 8 from the bonds with fluorine and 2 from the lone pair. This is quite possible for sulfur because it has easy access to its 3d-orbitals, which means that it can expand its octet and accomodate more than 8 electrons.
You determine sulfur's hybridization by counting the number of regions of electron density that surround it. Remember, a region of electron density is a bond (single, double, and triple bonds count as one region of electron density) or a lone pair of electrons.
In this case, sulfur forms 4 single bonds and has 1 lone pair, which means that its steric number, which is the name given to the number of regions of electron density, is equal to 5.
The steric number will give you the number of hybrid orbitals that the atom uses. In this case, sulfur will use five hybrid orbitals formed from
- the 3s-orbital;
- three 3p-orbitals;
- one 3d-orbital
The central atom will thus be


