Question #607b1
1 Answer
Explanation:
So, you know that the gas is sealed in a container. This will tell you two important things
- the amount of gas remains unchanged;
- the volume of the container remains unchainged
Now, the idea here is that the pressure of a gas is proportional to its temperature if volume and number of moles, which represents the amount of gas, remain constant - this is known as Gay Lussac's Law.
This means that if you increase the temperature of the gas, its pressure will increase as well. Likewise, if you decrease the temperature, its pressure will decrease as well.
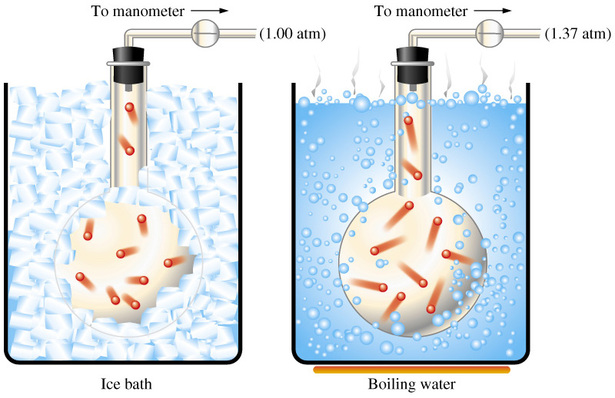
In your case, you know that the temperature is increased from
Mathematically, Gay Lussac's Law is written like this
#P_1/T_1 = P_2/T_2" "# , where
Plug in your value to get
#P_2 = T_2/T_1 * P_1#
#P_2 = (475color(red)(cancel(color(black)("K"))))/(298color(red)(cancel(color(black)("K")))) * "111 kPa" = "176.93 kPa"#
You need to round the answer to three sig figs
#P_2 = color(green)("177 kPa")#

