Question #a198a
1 Answer
Explanation:
You're dealing with the calcium atom,
Calcium is located in period 4, group 2 of the peridoc table and has an atomic number equal to
This means that a neutral calcium atom will ahve a total of 20 electrons surrounding its nucleus. These electrons are distributed on specific energy levels located at various distances from the nucleus.
More specifically, the higher its energy level, the further away from the nucleus the electron will be.
Calcium's electron configuration is
"Ca: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(4)s^2
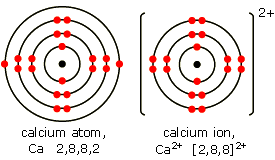
Now, the electrons located on the outermost energy level are the atom's valence electrons. The electrons that calcium loses to form the calcium cation,
 http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/atomic/ionicrev2.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/atomic/ionicrev2.shtml
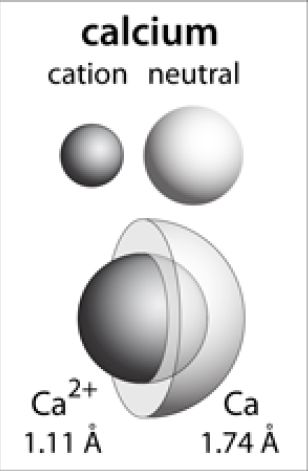
Now, because calcium loses the electrons located furthest from the nucleus, the calcium cation will have a smaller atomic radius than the calcium atom.
That happens because once the cation is formed, the outermost electrons will be on the third energy level,
"Ca"^(2+): 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6
Since the third energy level is closer to the nucleus than the fourth energy level, the atomic radius of the calcium cation will indeed be smaller than that of the calcium atom.
 http://www.atomsinmotion.com/book/chapter3/ions
http://www.atomsinmotion.com/book/chapter3/ions

