How do you write the electron configuration for "H", "H"_2, "H"_2^-, and "H"_2^(2-) and calculate their bond order?
1 Answer
Hydrogen atom's valence orbitals, before bonding, include every orbital, and all are the same energy for a specific
HAVING MORE THAN ONE ELECTRON SPLITS ENERGY LEVELS
When introducing more electrons into the system, i.e. with another hydrogen wanting to bond, the repulsion splits the energy levels of the AOs of hydrogen.
They start out where each
MOLECULAR ORBITAL THEORY
The basic tenant of Molecular Orbital Theory (MO Theory) is that the number of MOs formed by a linear combination of atomic orbitals (LCAO) is equal to the number of AOs used.
The energy splitting caused by electron/electron repulsion generates two MOs due to the one
The
MO DIAGRAM FOR DIATOMIC HYDROGEN MOLECULE & ION
Now, let us draw the MO diagram for the
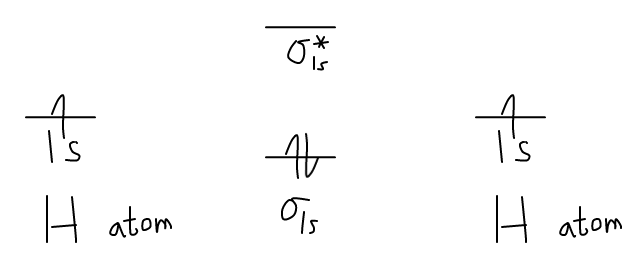
Each hydrogen contributes one electron, which therefore fills the lower-in-energy
Add one electron, and you will get

MO ELECTRON CONFIGURATIONS
Writing the MO electron configuration is fairly straightforward; read off of the diagram and put superscripts for how many electrons there are, analogous to the AO electron configuration.
The only difference is the symbol used to indicate the MO instead of the AO and some parentheses.
- For
"H" atom, it would just be1s^1 . - For
"H"_2 molecule, it would be(sigma_(1s))^2 . - For
"H"_2^(-) ion, it would be(sigma_(1s))^2 (sigma_(1s)^"*")^1 . - For
"H"_2^(2-) ion, it would be(sigma_(1s))^2 (sigma_(1s)^"*")^2 .
BOND ORDER DIRECTLY RELATES TO BOND STRENGTH
The Bond Order, then, is the number of bonding electrons minus the number of antibonding electrons, the total divided by
For
color(blue)("Bond Order") = ("No. of Bonding e"^(-) - "No. of Antibonding e"^(-))/2
= (2 - 0)/2 = color(blue)("1") which makes sense because
"H"_2 has one single bond.
For
color(blue)("Bond Order") = ("No. of Bonding e"^(-) - "No. of Antibonding e"^(-))/2
= (2 - 1)/2 = color(blue)("1/2")
The contribution of one electron in the antibonding orbital decreases the bond order, meaning that the bond in
If you're following the pattern, you should draw the MO diagram for
color(blue)("Bond Order") = ("No. of Bonding e"^(-) - "No. of Antibonding e"^(-))/2
= (2 - 2)/2 = color(blue)("0")
The contribution of one electron in the antibonding orbital decreases the bond order, meaning that the bond in
So weak, in fact, that since the bond order is

