Question #cb2ea
1 Answer
Explanation:
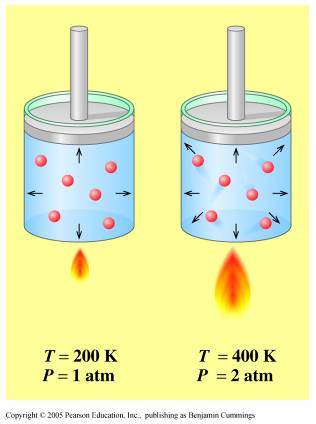
Notice that no mention of volume and number of moles was made, which means that you can assume that they remain constant.
When volume and number of moles are kept constant, pressure and temperature have a direct relationship - this is known as Gay Lussac's Law.
 https://elearning.kctcs.edu/bbcswebdav/users/kmuller0001/SoftChalk Filesl
https://elearning.kctcs.edu/bbcswebdav/users/kmuller0001/SoftChalk Filesl
Simply put, if temperature increases, pressure will increase as well. Likewise, if temperature decreases, pressure will decreases as well.
Mathematically, this is written as
P1T1=P2T2 , where
Now, the temperature of the gas increases from
Plug in your values and solve for
P2=T2T1⋅P1
P2=450K320K⋅1.5 atm=2.1 atm

