Question #1f3e8
2 Answers
Hybridization is
Explanation:
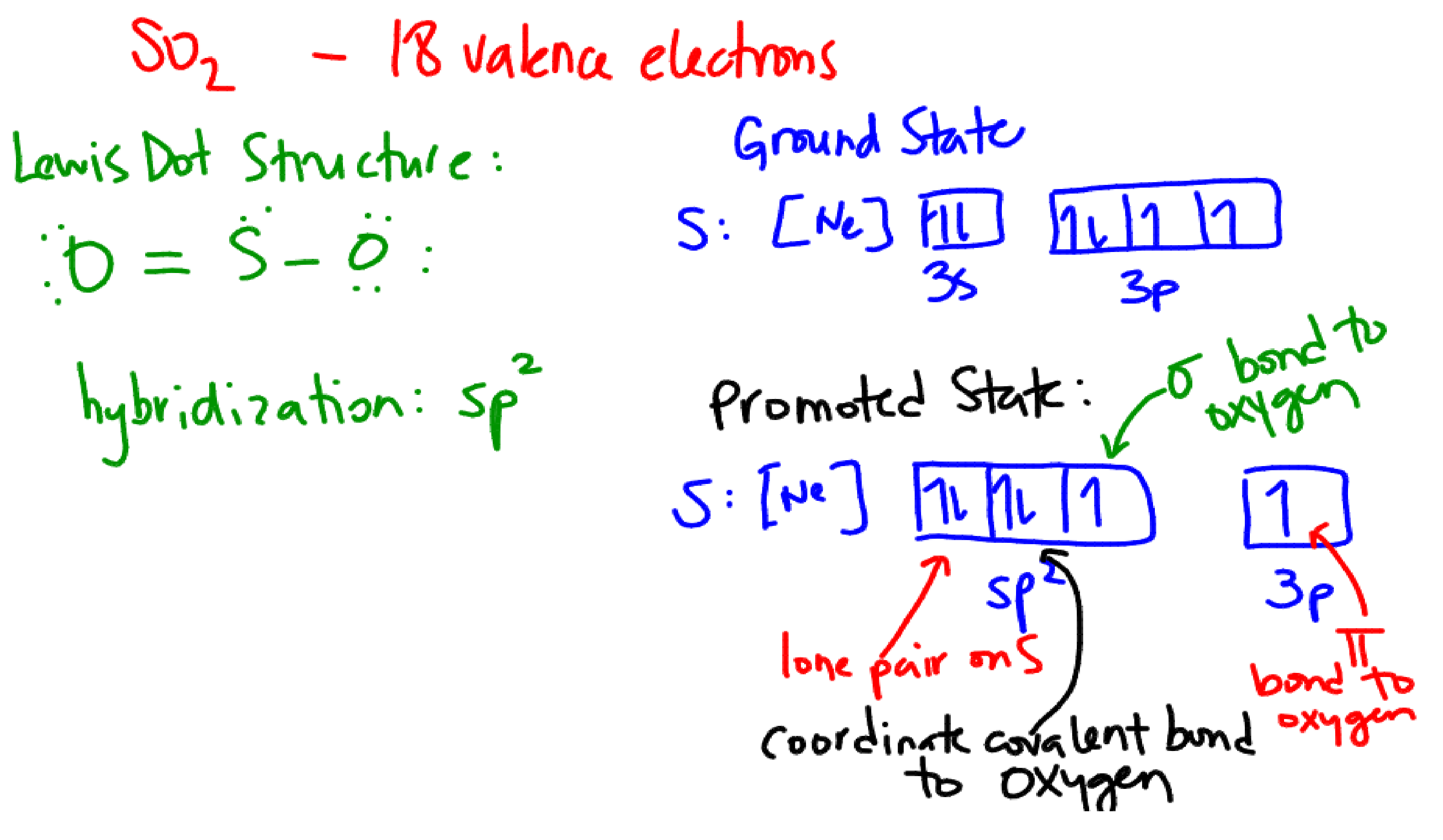
Explanation:
The electron pairs arrangement around sulfur is trigonal planar with a bond angle of around

For sulfur to achieve this geometry the orbitals
For the oxygen atoms, we should consider the hybrid form of
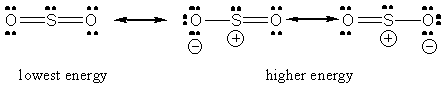
In the hybrid form the electron pairs arrangement around oxygen is also trigonal planar with a bond angle of around
For oxygen to achieve this geometry the orbitals
Images source: http://bilbo.chm.uri.edu/


