Question #cbbe3
2 Answers
I have given both the shortcut method and also explained in detail what makes the shortcut work
Explanation:
'~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
The wording; "diluted to" is stating the
Thus, expressed as a fraction we have:
'~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
What
To change this to percent we need to have
For a moment lets think of the
If we can retain this ratio but change the bottom number (denominator) to 100 we have our answer
Known:
So if we multiply
The
Explanation:
Let me offer a more chemical-oriented approach to this problem.
As you know, a solution's molarity is defined as the number of moles of solute divided by the volume of the solution - expressed in liters.
color(blue)("molarity" = c = "moles of solute"/"liters of solution" = n/V)
Now, the idea behind diluting a solution is that you can decrease its concentration by
- keeping the number of moles of solute constant**
- increasing the volume of the solution by increasing the volume of the solvent
The underlying principle of a dilution
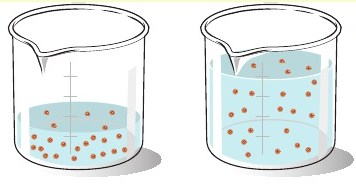
So, your initial sample has a total volume of
The target solution has a total volume of
The number of moles of solute present in these solutions will be
n_1 = c_1 * V_1" " and" "n_2 = c_2 * V_2
Since a dilution implies keeping the number of moles of solute constant
n_1 = n_2
you will get
color(blue)(c_1V_1 = c_2V_2) -> the equation for dilution calculations
Your goal is to determine the dilution factor, so you can rearrange the above equation to get
c_1/c_2 = V_2/V_1
Plug in your values to get
c_1/c_2 = (20 color(red)(cancel(color(black)("mL"))))/(0.25color(red)(cancel(color(black)("mL")))) = 80
Therefore, the initial solution was eighty times as concentrated as the target solution
c_1 = 80 xx c_2
And that's how you find the dilution factor. Simply divide the final volume of the solution by the initial volume of the sample
color(blue)("D"."F" = V_"final"/V_"initial") -> the equation for finding the dilution factor
A dilution factor of


