The atomic orbital of "H" that is both compatible and close enough in energy with the n = 2 atomic orbitals of "N" is the 1s.
The 1s atomic orbital of "H", with E = -"13.6 eV", is close enough in energy (less than "12 eV" away) to the 2p atomic orbitals of "N", with E = -"13.1 eV", that it can overlap with SOME of them.
In a nonlinear molecule, the 2p_y orbitals of nitrogen line up directly (head-on, colinear) with hydrogen---the y direction of the "N"-"H" bond is along the bond. The z direction is through the tip of the trigonal pyramid.
The MO diagram for "NH"_3 is:
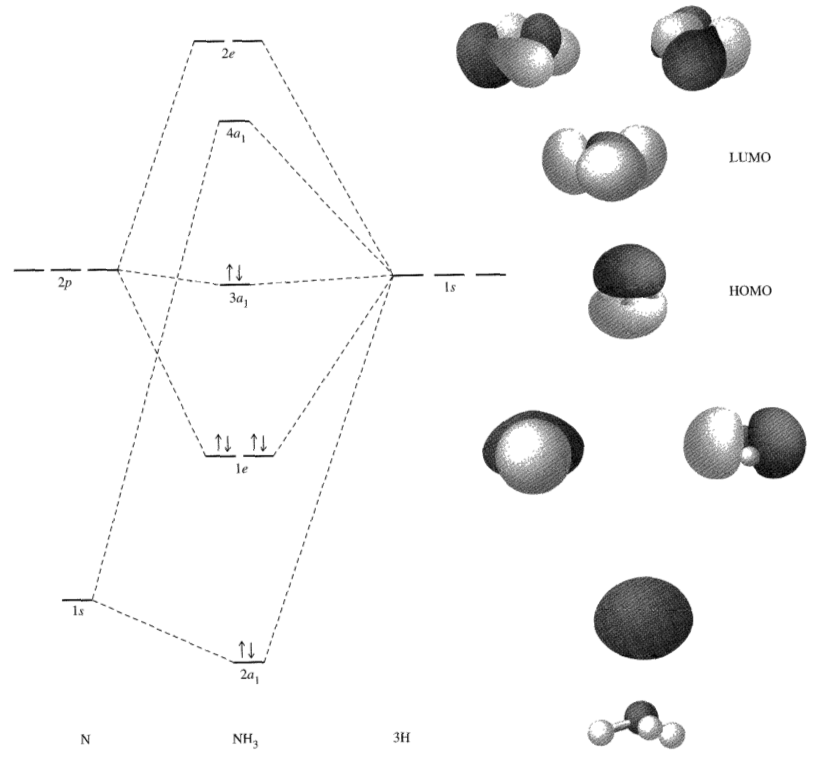 Inorganic Chemistry, Miessler et al., Ch. 5.4.4
Inorganic Chemistry, Miessler et al., Ch. 5.4.4
[Do note though that this diagram has a typo; the 1s atomic orbital of nitrogen should be a 2s.]
As you can see, the 2p atomic orbitals of "N" are indeed very close in energy with the hydrogen 1s atomic orbitals.
The nonbonding orbital holding the lone pair is the 3a_1, which ends up being contributed to mostly by the 2p_z of "N" and slightly by the 1s orbitals of the three "H" atoms as a group.
So, the "orbital overlap" diagram would look something like this:
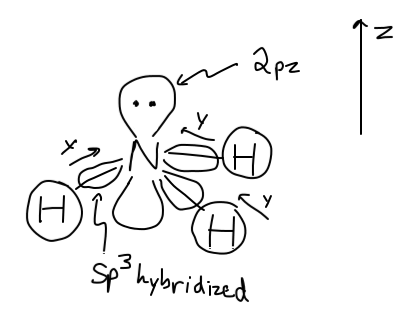
 Inorganic Chemistry, Miessler et al., Ch. 5.4.4
Inorganic Chemistry, Miessler et al., Ch. 5.4.4 

