Why is #3^@ > 2^@ > 1^@ > "methyl"# the trend for reactivity in an #"S"_N1# reaction but #3^@ < 2^@ < 1^@ < "methyl"# for #"S"_N2# reactions?
1 Answer
From an
In an
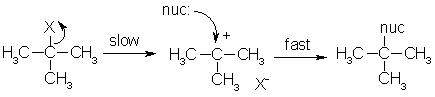
STABILIZATION OF THE INTERMEDIATE
Postulate: We know that if a compound is more stable, it tends to willingly stay in its current state. Similarly, the more stable the intermediate, the more favorably it stays in its current state once it forms.
The greater the number of electron-donating groups (e.g. alkyl) around the carbocation, the more electron density is donated into the electrophilic carbon, stabilizing it via hyperconjugation.
Hyperconjugation is an effect where the
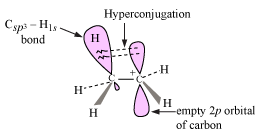
In general, spreading out electron density is stabilizing, almost like you would see in resonance structures of conjugated
Thus, the more alkyl groups around the electrophilic carbon, the more stabilized the carbocation intermediate, and the lower in energy it is.
Thus it can stay in that state for long enough that a weak nucleophile can still form a bond with it, making it more favorable for the intermediate to form (remember that
Thus, we have the
This is a thermodynamic effect, as it affects the energy of the intermediate and thus the Gibbs' free energy of the reaction's first step.
AN ADDITIONAL KINETIC EXPLANATION
There can be more than one angle to this.
The strength of the
BASICITY OF LEAVING GROUP
Let us assume comparisons between carbocations of the same kind (
The stronger the basicity of the leaving group, the stronger the
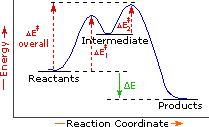
Since reactivity is a kinetic factor, and since a more stable reactant is associated with a larger activation energy, it correlates with a slower rate-limiting step, favoring
(It's like trying to drive up a very tall and steep hill, but failing and realizing you need to start further up the hill.)
STERIC BULK
When I say this, I am referring to both the leaving group and everything around the electrophilic carbon.
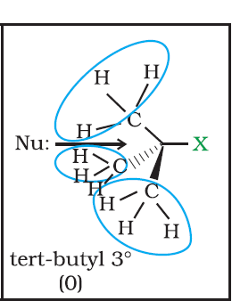
If the leaving group is bulky (large), it's going to be quite favorable for it to leave because of the steric clash; it's just too "cramped", like you would be amidst a busy New York crowd.
If the stuff around the carbocation is crowding the leaving group, that also facilitates the
Both work along similar lines, destabilizing the reactant and thus decreasing the activation energy.
Thus,
This is a kinetic effect, since it is associated with the activation energy.

