What is the product from the reaction of 4-cyclohexylbenzene with fuming sulfuric acid?
1 Answer
Jan 26, 2017
I would expect the product to be 4-cyclohexylbenzenesulfonic acid.
Explanation:
Sulfonation of a benzene ring is a standard electrophilic aromatic substitution reaction.
It is easily carried out by gentle warming of the aromatic compound with fuming sulfuric acid at 40 °C for about 30 min.
The electrophile is probably the protonated
The mechanism is as shown below for benzene.
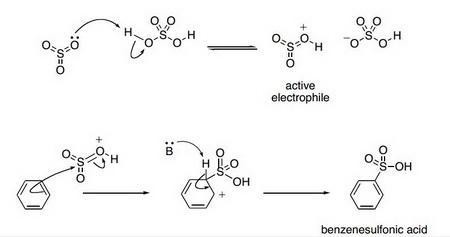
(From StudyBlue)
The cyclohexyl group is an ortho/para director.
Its bulk should hinder attack at the ortho position.
I predict that the major product will be 4-cyclohexylbenzenesulfonic acid.
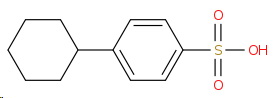
(Structure by OPSIN)

