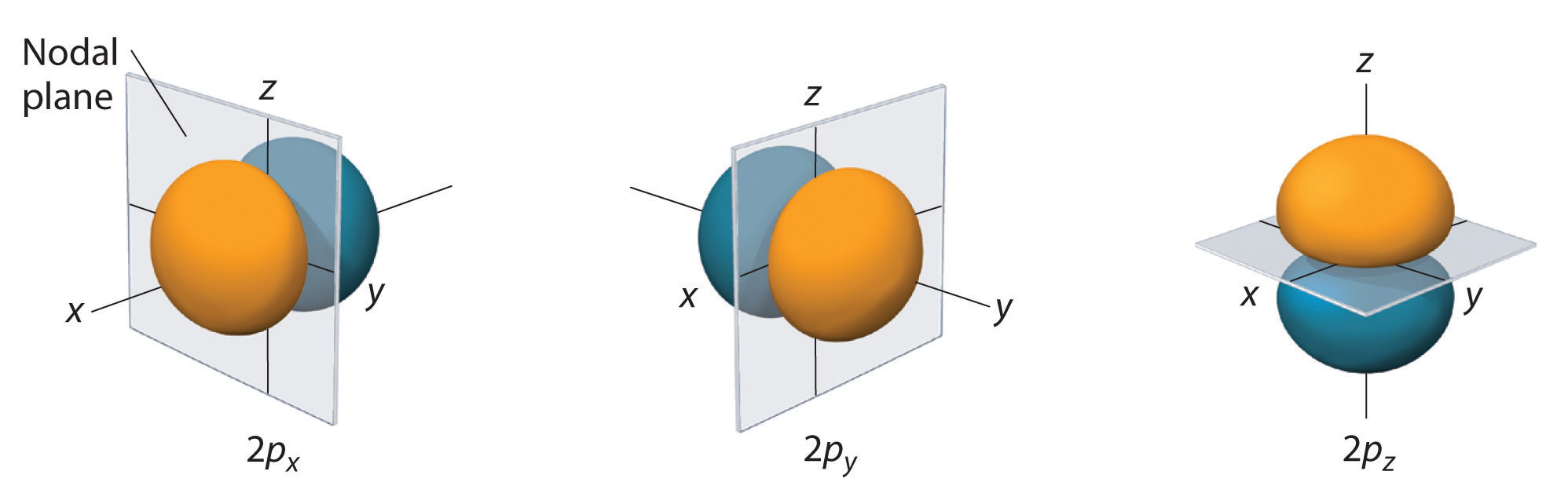
What is the formula for calculating a nodal point?
1 Answer
There is none. You need the wave function for the particular orbital, which is not readily available unless the atom is hydrogen...
In the case of hydrogenic atoms, i.e. one-electron atoms, the wave functions are available in most physical chemistry textbooks up through
As a simple but not undercomplicated example, consider one of the
The hydrogenic atom wave function for the
#psi_(2pz) = R_(21)(r)Y_(1)^(0)(theta,phi)#
#= 1/(4sqrt(2pi)) (Z/a_0)^(3//2) sigmae^(-sigma//2)costheta# ,where:
#sigma = Zr//a_0# .#Z# is the atomic number.#a_0 = "0.0529177 nm"# is the Bohr radius.#r# is the radial distance away from the nucleus.#R_(nl)(r)# is the radial component of the wave function.#Y_(l)^(m_l)(theta,phi)# is the angular component of the wave function.
The radial nodes are where
Since nonzero constants are clearly never zero, we just have to pick out the functions that have
#R_(21)(r) prop sigmae^(-sigma//2)#
#Y_(1)^(0)(theta,phi) prop costheta#
RADIAL NODES?
So, solving for the radial nodes, with
#(Zr)/(a_0)e^(-Zr//2a_0) = 0#
This exponential never goes to zero except at
#=> (Zr)/(a_0) = 0#
From here we can realize that there are no radial nodes in the
The only solution to this is
ANGULAR NODES?
Solving for the angular nodes,
#costheta = 0#
And this only nonredundantly applies for
So, in this domain,
Furthermore, there is no
#"Probability Density of 2p"_z#
#= int_(0)^(2pi) int_(0)^(pi) int_(0)^(oo) psi_(2pz)^"*"psi_(2pz)r^2dr sinthetad theta d phi#
#= color(red)(1/(4sqrt(2pi)) (Z/a_0)^(5) int_(0)^(2pi) d phi) int_(0)^(pi) cos^2theta sintheta d theta int_(0)^(oo) r^2e^(-Zr//a_0)dr#
#= color(red)((2pi)/(4sqrt(2pi))(Z/a_0)^(5)) int_(0)^(pi) cos^2theta sintheta d theta int_(0)^(oo) r^2e^(-Zr//a_0)dr#
Having no dependence on the value of
Therefore, the angular node is a plane perpendicular to the