Is there a direct proportionality between base strength and ionic radius for anions in aprotic solvents?
1 Answer
Let's look at a fairly straightforward example. Consider the hydrohalogenic acids,
RADIUS AND BOND LENGTH VS. ACID/BASE STRENGTH
The ionic radii of the halides go as follows:
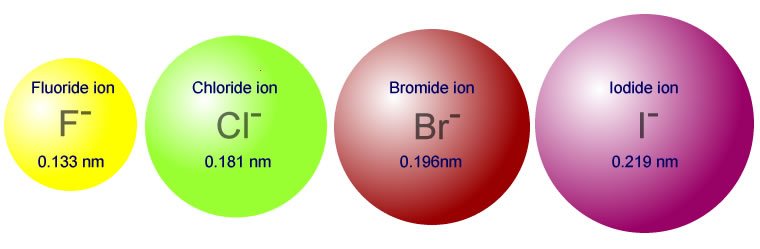
What this leads to is the following trend in
#r_("H"-"I") > r_("H"-"Br") > r_("H"-"Cl") > r_("H"-"F")#
- The longer the
#"H"-"X"# bond length, the weaker the#"H"-"X"# bond, and the stronger the acid. - The stronger the acid, the weaker the conjugate base.
So, the strongest acid here is
CHARGE DENSITY VS. BASE STRENGTH
This correlates with a decreasing charge density as well.
Since
Iodide's higher polarizability means it is harder to localize (to concentrate into one spot) the electron density in between
(Another interpretation is that the orbital overlap becomes less stabilizing because not all the electron density is in the right spot for the resultant molecular orbital to be as stable as possible.)
Hence,
So, in this sense, there is a direct correlation where the increasing charge density leads to an increasing basicity.
(Note that this only holds true for nucleophilicity in aprotic solvent.)

