What is the hybridization and symmetry of #["Cu"_2"Cl"_8]^(4-)#?
1 Answer
I got its symmetry as
DISCLAIMER: Kind of long answer.
I think you are asking about
A similar compound is
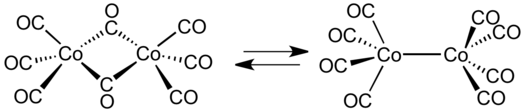
(
Similarly, dicopper octachloride anion,
ORBITAL "HYBRIDIZATION"
It's not easy to say what its hybridization is.
The paper states it is a distorted trigonal bipyramidal molecular geometry around the copper with "one axial and one equatorial chlorine" atoms bridging the two not-bonded copper atoms. One could say it is
Assuming the
- The bridging chlorine atoms can be said to each use their
#3p_x# atomic orbital to bond with each copper's#3d_(xy)# orbitals. - Probably, each chlorine can use its
#3p_z# orbital for some weak#pi# -bonding with the coppers'#3d_(yz)# orbitals (two-lobe same-sign overlap of the#d# orbital's top two lobes).
Below I've depicted the
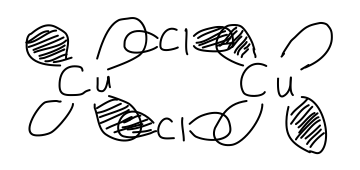
As the configuration of each copper and its respective three terminal chlorine atoms resembles
POINT GROUP SYMMETRY
As for the symmetry (which I'm assuming means point group symmetry), we can start from thinking about the molecule from a different angle.
PRINCIPAL AXIS
First, find the principal axis. By convention that is the z-axis as well.
If you look down the
Unfortunately the bridging chlorines break that symmetry.
The alternative is the axis through the plane of the screen, which rotates the molecule, the whole time remaining upon the plane of the screen - that is a
MIRROR/REFLECTION PLANES
Next, you should consider whether the molecule has a mirror plane (at least one coplanar, and possibly one perpendicular to the principal axis).
In fact, it does.
-
The
#(mu_2-"Cl")_2"Cu"_2# plane (the plane of the depicted orbital overlap) is a horizontal reflection plane,#sigma_h# , because it is perpendicular with the#z# -axis, and it is labeled#sigma_h# to indicate that -
Since this molecule is staggered in its most stable form, it does not have a vertical mirror plane,
#sigma_v# , intersecting the bridging#"Cl"# atoms and bisecting the left/right halves of the molecule.
POINT OF INVERSION
Another thing to consider is that it has a center of inversion,
So, you reflect through the
PUTTING IT ALL TOGETHER
Now, for these reasons:
- The principal axis is a
#C_(color(red)(2))# axis. - There is a
#sigma_color(red)(h)# horizontal reflection plane perpendicular with the principal rotation axis. - There is NO
#C_2# axis perpendicular to the principal axis, making this NOT a dihedral (#D# ) group. - There IS a center of inversion.
Because of this, this should be classified as

