What is a node when it comes to electron density, waves, and wave functions?
1 Answer
Aug 25, 2016
A node is a region of zero electron density. That means you cannot find electrons at a node.
Visually, on a wave, it would be the point on the wave where the concavity changes. In other words, it is the zero-crossing.
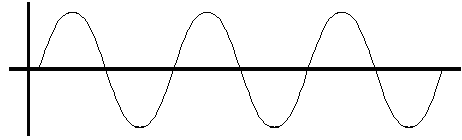
Formally, it is when the wave function is
#psi_("atomic orbital") = 0#
except at zero distance away from the nucleus.

