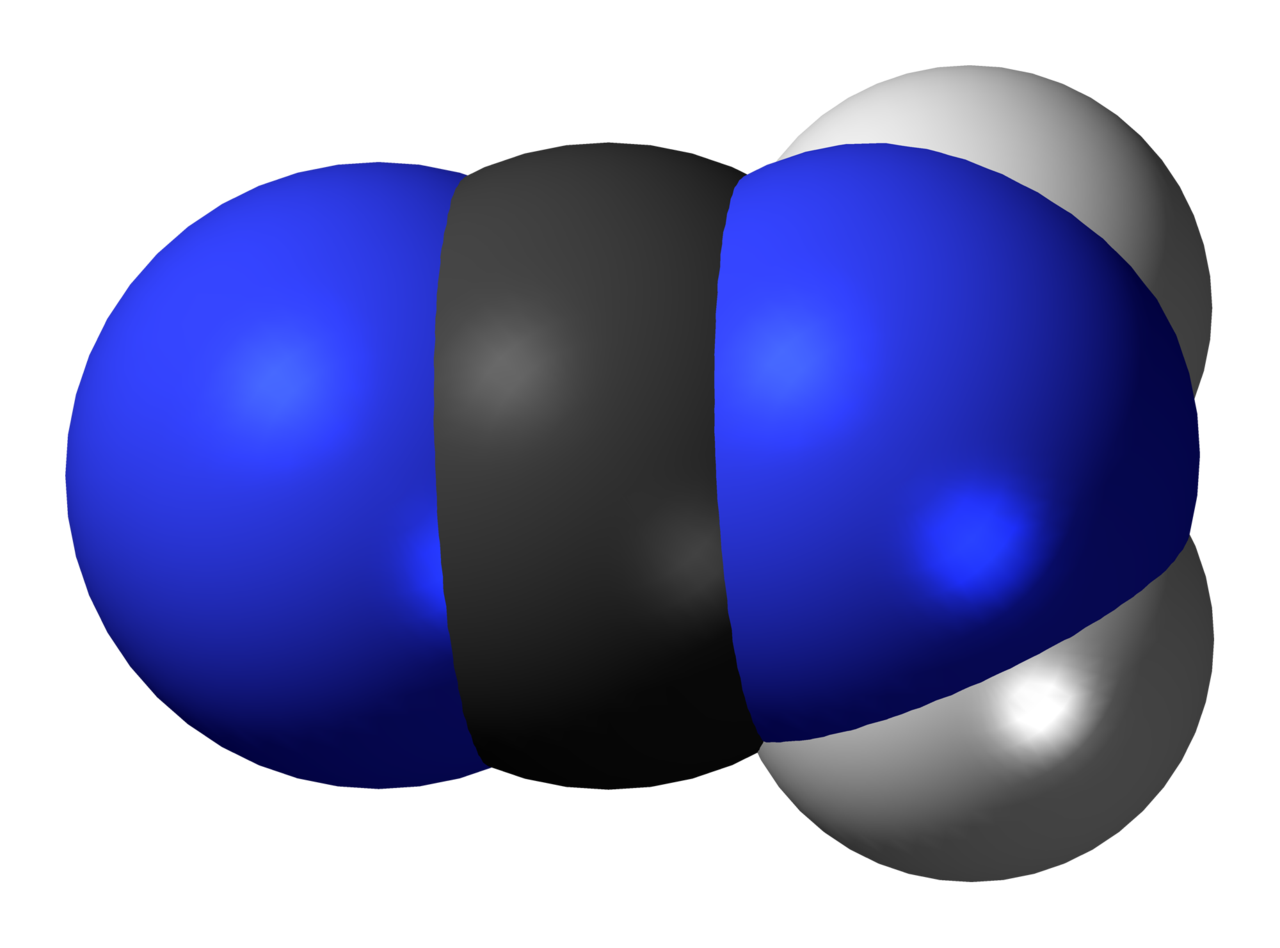
Question #75b36
1 Answer
Here's how you can do that.
Explanation:
The thing to remember about molecular geometry is that you must always look for the number of regions of electron density that surround an atom, which is called the steric number.
A region of electron density is simply
- a covalent bond
#-># here single, double, and triple bonds count as one region of electron density- a lone pair of electrons
Take a look at the carbon atom. This atom is surrounded by two regions of electron density because
- it forms a triple bond with the leftmost nitrogen atom
- it forms a single bond with the rightmost nitrogen atom
- it doesn't have lone pairs of electrons attached
Now, the steric number gives you the hybridization of the atom. In this case, a steric number equal to
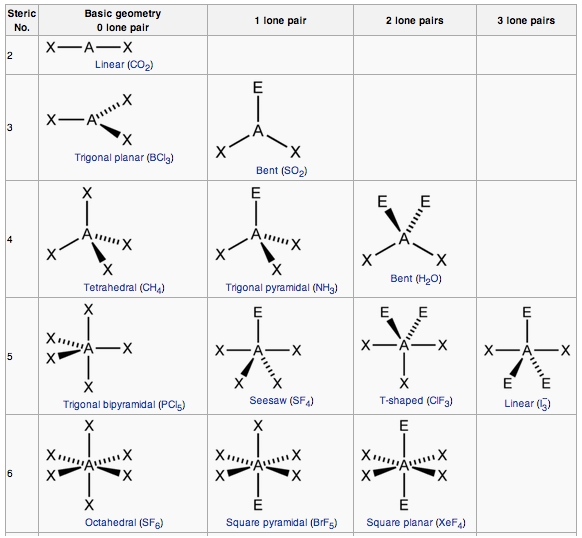
This is consistent with the
Now focus on the central nitrogen atom, i.e. the rightmost one. This atom is surrounded by four regions of electron density because
- it forms a single bond with the carbon atom
- it forms two single bonds with the two hydrogen atoms
- it has one lone pair of electrons attached
For this atom, the steric number is equal to
This is consistent with the
It's worth mentioning that the actual bond angle surrounding the nitrogen atom is
Here's how a cyanamide molecule would look like (the major resonance structure)