What are the symmetry elements of the isomers of dichlorocyclobutane?
1 Answer
Since you have not specified which isomers, I will simply list four of them (assuming they are on different carbons):
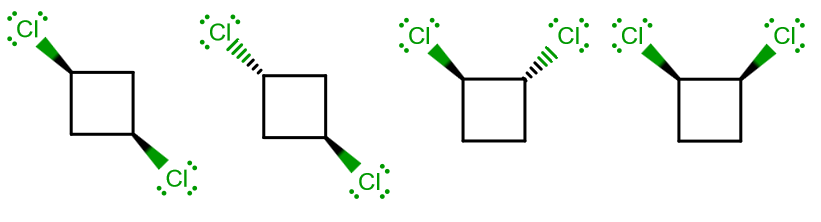
From left to right, we have the cis-1,3, trans-1,3, trans-1,2, and cis-1,2 isomers. I will label them from left to right, isomers (1), (2), (3), and (4).
(1):
(2):
(3):
(4):
So basically:
(1): Identity, one rotation axis, two reflection planes
(2): Identity, one rotation axis, one point of inversion, one reflection plane
(3): Identity, one rotation axis
(4): Identity, one reflection plane
DISCLAIMER: Long answer! Possibly difficult to visualize...
The possible, primary symmetry elements overall (for any compound) are:
#E# , the identity element, for completeness's sake.#C_n# , the principal rotation axis, where a rotation of#360^@/n# about this axis returns the original molecule. This is defined to require the largest rotation angle to return the original molecule.
We define this to be along the#bb(z)# axis.#C_n'# , any other rotation axis, defined similarly; just something that isn't the previously-defined#C_n# . It could be perpendicular.#sigma_v# , the vertical reflection plane, colinear with the principal rotation axis#C_n# . Generally, crosses through atoms.#sigma_h# , the horizontal reflection plane, generally on the plane of a cyclic molecule that is perpendicular to the#C_n# axis.
If no#C_n# axis exists except for the trivial#C_1# (#C_1 = E# ), then we assign a reflection plane as#sigma_h# .#sigma_d# , the dihedral reflection plane, generally in between two atoms, bisecting a bond (this is not common unless#sigma_v# and#sigma_h# are also identified).#i# , the point of inversion. Basically you take the coordinates#(x,y,z)# and swap them with the coordinates#(-x,-y,-z)# .
Obviously, all four isomers have
ISOMER (1)
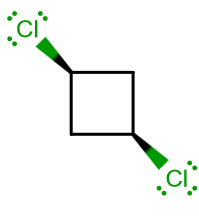
- There is one
#C_n# axis through the ring, through the plane of the screen. That is a#bb(C_2)# principal rotation axis, along the#z# -axis. Therefore, the plane of the ring is the#xy# -plane. - There is one
#bb(sigma_v(xz))# vertical reflection plane bisecting the molecule through carbon-1 and carbon-3 (perpendicular to the ring). - There is one more
#bb(sigma_v(yz))# vertical reflection plane bisecting the molecule through carbon-2 and carbon-4 (perpendicular to the ring).
That, by the way, assigns this molecule to a
ISOMER (2)
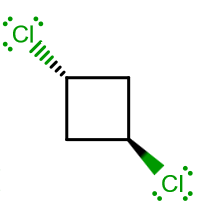
Compared to (1), there is no longer a
- There is one
#bb(C_2)# axis bisecting the molecule through carbon-2 and carbon-4. We now define that as its#z# -axis. Let the plane of the molecule be the#yz# -plane, then (so that the#x# axis is through the plane of the screen). - There is one point of inversion,
#bb(i)# . Try taking carbon-1 and carbon-3, and switching their coordinates. Your chlorines would exactly swap places. - There is one
#bb(sigma_h(xz))# vertical reflection plane bisecting the molecule through carbon-1 and carbon-3 (perpendicular to the ring). Since it is perpendicular to#C_2# , it is still considered "horizontal". We've just reoriented our axes.
That, by the way, assigns this molecule to a
ISOMER (3)
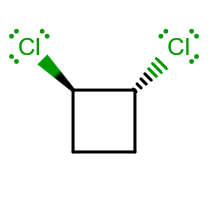
Compared to (2), there is no point of inversion
Using the same reasoning as in (1) and (2):
- There is a
#bb(C_2)# principal rotation axis bisecting the#C_1-C_2# bond, and we define that as the#z# -axis since#n# is as low as possible (#C_1 = E# ).
And I think that's it... I cannot find any reflection planes. There is no point of inversion as I mentioned before, since the chlorines are both on the same side.
So this is, by the way, assigned to a
ISOMER (4)
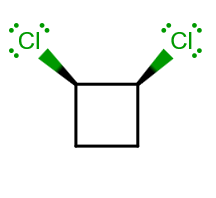
Compared to (3), there is actually a reflection plane, but no
- All I see is a
#bb(sigma_h)# reflection plane, since there is no#C_n# axis I can find. This is bisecting the molecule through the#C_1-C_2# bond (perpendicular to the plane of the screen).
So this is, by the way, assigned to the
Does this make sense? It may require you to pull out a model kit to visualize this.

