Question #85ebf
1 Answer
hydroxyl (Alcohol) and a carbonyl (
Explanation:
A Carbohydrate has the general formula
So it has to have two times as many hydrogens as there are as many oxygens and another number of carbons
All carbohydrates must also be either a polyhydroxy aldehyde or a polyhydroxy ketone. In both, you have a hydroxy, which is an alcohol. SO the carbohydrate would need to have an oxygen bonded to a hydrogen somewhere in the molecule. It would look like:
Before hydroxy, it says poly, so it needs a few hydroxyls. This also means that the Carbon Chain has to be bigger than just 1 or 2 Carbons Long.
You also have either a Aldehyde or a Ketone. An Aldehyde is an oxygen double bonded to a Carbon at the end of the chain, and a hydrogen is also bonded to the same Carbon.
This looks like:
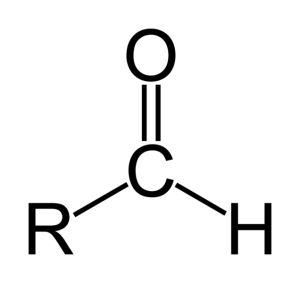 https://biochem1oh1.files.wordpress.com/2013/04/636px-aldehyde2png?w=300
https://biochem1oh1.files.wordpress.com/2013/04/636px-aldehyde2png?w=300
(Where R is rest of Chain)
This can also be a Ketone which is an Oxygen double bonded to a Carbon anywhere in the middle of the chain.
This looks like:
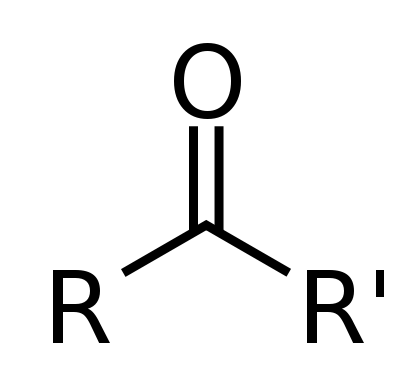 https://classconnection.s3.amazonaws.com/230/flashcards/1322230/png/screen_shot_2012-09-04_at_105325_pm1346813628187png
https://classconnection.s3.amazonaws.com/230/flashcards/1322230/png/screen_shot_2012-09-04_at_105325_pm1346813628187png
(Where R' may be a different rest of chain to R)
Both of these have a
So the two functional groups a carbohydrate needs are a hydroxyl (alcohol) and a Carbonyl (

