Question #6b789
1 Answer
Here's why that is the case.
Explanation:
For starters, a calcium has an atomic number equal to
The electron configuration of a neutral calcium atom looks like this
#"Ca: " 1s^2 2s^2 2p^6 3s^3 3p^6 color(red)(4)s^color(blue)(2)#
As you can see, a neutral calcium atom has
In this case, calcium's outermost energy level, which corresponds to
Now, in order to complete its octet, i.e. get
- the same number of protons as the neutral atom
- two electrons less than the neutral atom
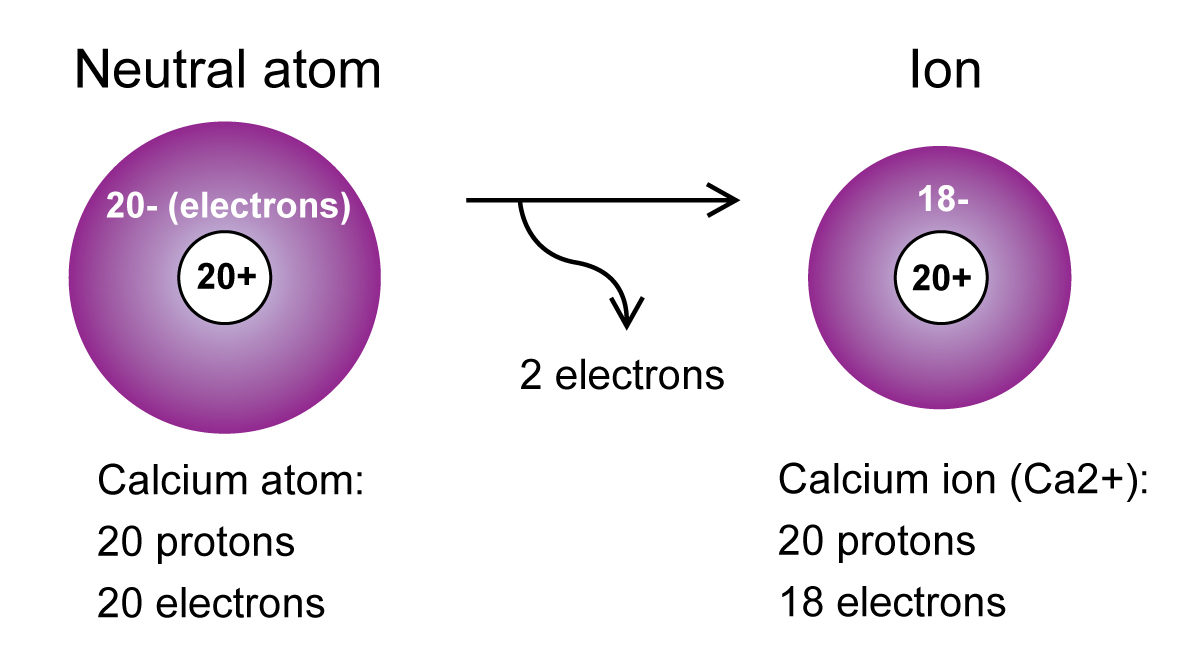
The electron configuration of the
#"Ca"^(2+): color(white)(.) 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6#
The outermost energy shell for a calcium ion has
#overbrace("2 e"^(-))^(color(blue)("in the 3s orbital")) + overbrace("6 e"^(-))^(color(blue)("in the 3p orbitals")) = "8 e"^(-) -># a complete octet

