If I have two atoms that come in with one half-filled orbital each, do they make a #sigma# bond?
1 Answer
Well, yeah. If you have two atoms each with a singly-occupied atomic orbital, if they come together and have ideal orbital overlap to form molecular orbitals, the number of electrons is conserved and the number of orbitals is conserved.
If each atom comes in with one electron in an atomic orbital, then the molecule must have a total of two electrons contributed in total from those two atoms (and two molecular orbitals result, one bonding and one antibonding).
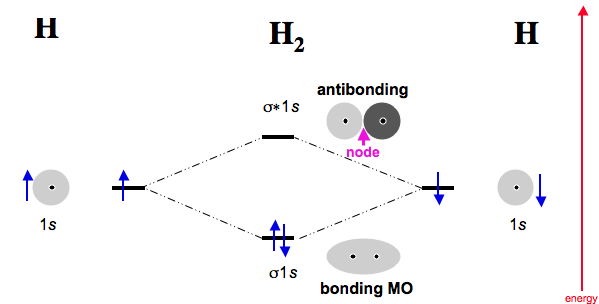
(Here, the bonding orbital is the
#sigma_(1s)# , and the antibonding,#sigma_(1s)^"*"# .)
If two electrons, one in each atomic orbital, start off unpaired, they become paired (if and only if the degeneracy
And in fact, for the first covalent bond formed in any molecule, one in-phase and one out-of-phase
So, by the definition of degeneracy, there is only one sigma molecular orbital in its own energy level, and thus the two electrons have to go in the same
Footnote:

