Question #4bb49
1 Answer
Mar 5, 2017
Explanation:
Hydrogen is located in period 1, group 1 of the Periodic Table of Elements and has an atomic number equal to
This implies that the electron configuration of a neutral hydrogen atom must account of a single electron.
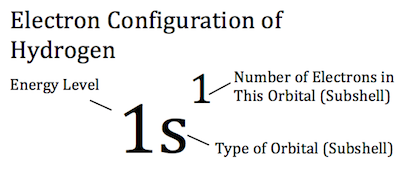
This electron will be located on the first energy level, in the
Therefore, the electron configuration for hydrogen will be
"H: " 1s^1