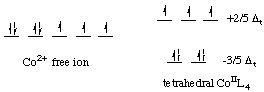What kinds of electron configurations can we get with octahedral and tetrahedral "Co"^(2+) complexes?
1 Answer
WARNING! Long answer! The octahedral complexes are either
Explanation:
Almost all the tetrahedral complexes are
Crystal Field Theory
In an octahedral field, the the five degenerate
- two high-energy orbitals, designated as
e_g - three low energy orbitals, designated as
t_2g .

The difference between the energy levels is
The value of
In a tetrahedral field, the energy levels are reversed.
The
- three
t_2g high-energy orbitals - two
e_g low-energy orbitals
Octahedral
The electron configuration of
When we have a strong-field ligand like
The extra electrons occupy the lower
(Adapted from chemed.chem.purdue.edu)
When we have a weak-field ligand like
The electrons fill the orbitals in a Hund's Rule type of order and pair up only when they have no other choice.

A high-field ligand like
Thus,
Tetrahedral

Thus, tetrahedral