Question #5ed28
1 Answer
Mar 19, 2017
Here's what I get.
Explanation:
For geometric isomerism, you need restricted rotation, usually caused by a double bond or a ring.
(i) 2,3-Dichlorohex-2-ene
2,3-Dichlorohex-2-ene
Two geometric isomers. The two
Distinguish by dipole moment. The cis isomer has a dipole moment. The trans isomer should have µ ≈ 0.
(ii) 2,2-Dimethylpropane
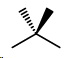 2,2-Dimethylpropane
2,2-Dimethylpropane
No geometric isomers.
(iii) Butan-1,3-diol
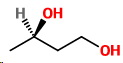 Butan-1,3-diol
Butan-1,3-diol
No geometric isomers, but there are optical isomers.
(iv) 2,2-Dihydroxy-1-phenylpentan-3-one
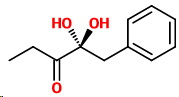 2,2-Dihydroxy-1-phenylpentan-3-one
2,2-Dihydroxy-1-phenylpentan-3-one
No geometric isomers.

