An outer orbital complex uses #"d"# orbitals from the #(n)# shell to form #"sp"^3"d"^2# hybrid orbitals for bonding.
Inner orbital complexes
#["Co"("NH"_3)_6]^"3+"# is an inner orbital complex.
The electron configuration of #"Co"^"3+"# is #"[Ar] 3d"^6#.
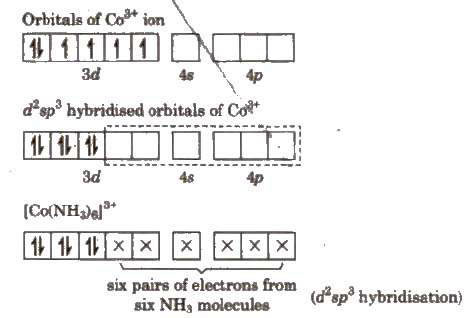
The #"3d, 4s"# and #"4p"# orbitals are close in energy, so the ion can easily form six #"d"^2"sp"^3# hybrid orbitals for bonding to the #"NH"_3# ligands.
This is an inner orbital complex because it uses the "inner" #"3d"# orbitals for bonding.
Outer orbital complexes
#["CoF"_6]^"3-"# is an outer orbital complex.
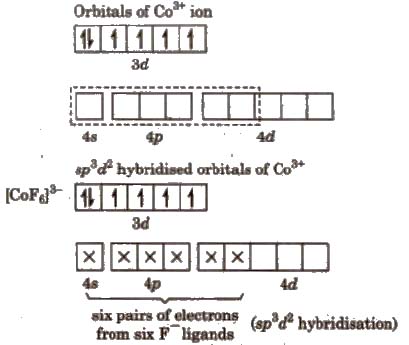
Here, the empty #"4s, 4p"# and two of the #"4d"# orbitals can form six #"sp"^2"d"^3# hybrid orbitals for bonding to the #"F"^"-"# ligands.
This is an outer orbital complex because it uses the "outer" #"4d"# orbitals for bonding.
For an excellent explanation of inner and outer orbital complexes, see this Socratic answer.



