Question #19845
1 Answer
The first step to solving such questions would be writing the chemical equations.
Explanation:
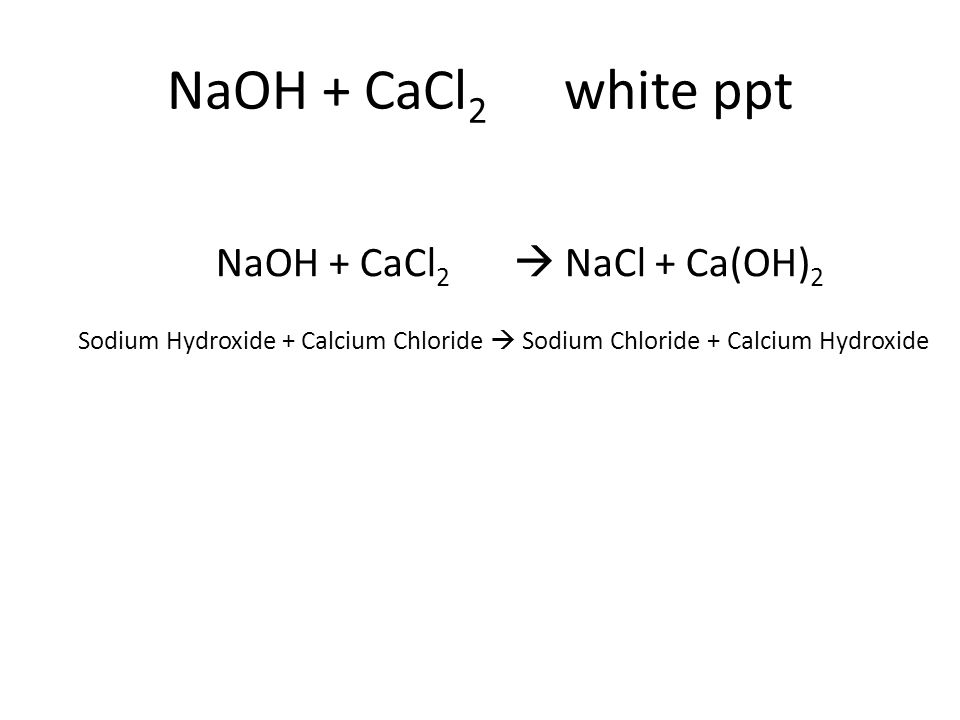
Given that there is
the amount of calcium chloride in moles is
By molar ratio, the amount of sodium chloride formed in moles is
The molar mass of sodium chloride is
The mass of sodium chloride formed is
Perhaps taking note of the units can help you to find answers; for example:
gives the answer is grams (the mass) and so on and so forth!


