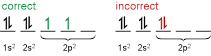Question #8086f
1 Answer
May 19, 2017
You mean 'Hund's Rule'? => every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin.
Explanation: