How do indicators work?
2 Answers
It is done by putting the indicator in the Acid or Base.
Explanation:
pH indicators detect the presence of
. They do this by reacting with
: they are themselves weak acids and bases. If an indicator is a weak acid and is colored and its conjugate base has a different
color, deprotonation causes a color change.
Well, see this old answer.
Explanation:
An indicator is a large, weak organic acid whose acid and base forms have distinctive colours. And we can represent this species by
The point is that
 imgarcarde.com
imgarcarde.com
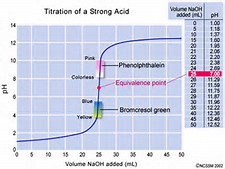
The graph shows (poorly) the titration curve of a strong acid, when titrated by a strong base. Because the rise in


