What does it mean that a substance absorbs at #lambda_max#? How is absorbance related to concentration?
1 Answer
Well,
Beer's law states:
#A = epsilonbc# where:
#A# is absorbance.#epsilon# is molar absorptivity in#"L/mol"cdot"cm"# .#b# is the path length of the cuvette in#"cm"# .#c# is the concentration in#"mol/L"# .
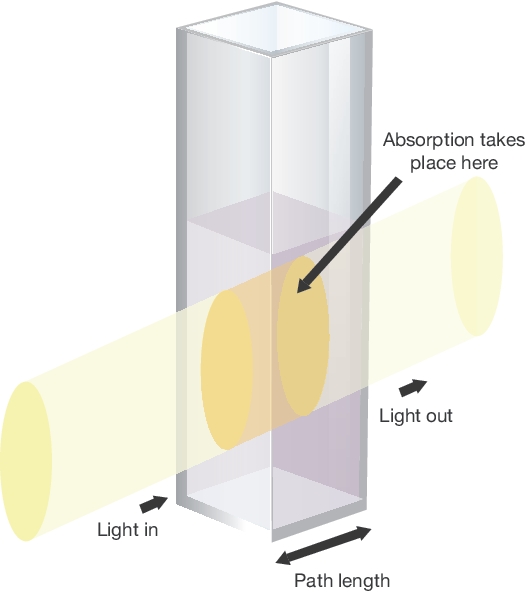
We simply have that the more stuff in the way of light, the more light is blocked and then absorbed.
Less stuff in the way
#-># lower concentration#-># less light absorbed.Lower amounts of light absorbed
#-># more light reflected#-># lighter colors (indicating lower absorbance).
Hence, as concentration decreases, absorbance must decrease for the same substance.
When you dilute, what happens to the concentration? What therefore happens to the absorbance? Should the solution get darker, or lighter as a result of a dilution?
(Hopefully you answered "it goes down", "it goes down", and "lighter".)

